In 1905, Friedrich Best presented a detailed pedigree of an inherited retinal condition referred to as vitelliform dystrophy, or Best’s disease. Best’s disease is an inherited dystrophy of the macula that primarily involves cells known as retinal pigment epithelium (RPE).

Best’s typically affects both eyes and presents itself either in childhood or early adulthood. Visual acuity is usually minimally affected early on in the course. As the condition progresses, the vision can slowly begin to deteriorate. The rate of progression or the overall amount of progression is difficult to predict. The rate of progression may be also be asymmetric, with one eye progressing at a different rate than the other. Some patients may notice the development of scotoma, or “blind spot”, in their central vision as the condition progresses. Other patients may not progress to later stages or experience vision loss. Loss of peripheral, or side vision, is not expected with Best’s.
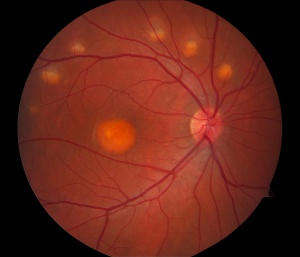
The classic exam finding in Best’s is a circular yellow lesion in the macula. This lesion resembles an “egg-yolk”, and is often referred to as such by ophthalmologists. As the condition progresses, the yellow material begins to break up and the pigmentation of the macula attains a more mottled appearance. This is often referred to as a “scrambled egg” appearance. After many years, there may be evidence of cell loss in the macula, which can negatively impact the visual acuity. In a relatively small proportion of cases, a complication can occur in which abnormal blood vessels grow underneath the macula and begin to leak fluid and/or blood into the macula. This is known as choroidal neovascularization (CNV), and can be vision threatening. Fortunately , CNV can be treated effectively with medications that are injected into the eye as part of a straightforward and low-risk office procedure. Typical signs of CNV would include distortion or blurring of the vision, and it is important to notify your doctor of any sudden changes in vision.
Diagnostic testing is sometimes used to confirm the diagnosis. The electro-oculogram (EOG) is universally abnormal in Best’s, and can be a valuable confirmatory test. Fluorescein angiography and optical coherence tomography can be valuable tests to better evaluate the macula and to also look for the development of CNV. Genetic testing for Best’s is now possible as well.
There is no established medical or surgical management for Best’s disease. In patients who develop CNV as a secondary complication, existing treatment options are effective. Future avenues of therapy hold significant promise, but are in their early stages of development. Stem cell based therapies, for example, have the potential to help restore healthy cells that may have been lost during the disease progression.
10/29/15
 Daniel D. Esmaili, MD
Daniel D. Esmaili, MD
Retina Vitreous Associates Medical Group

