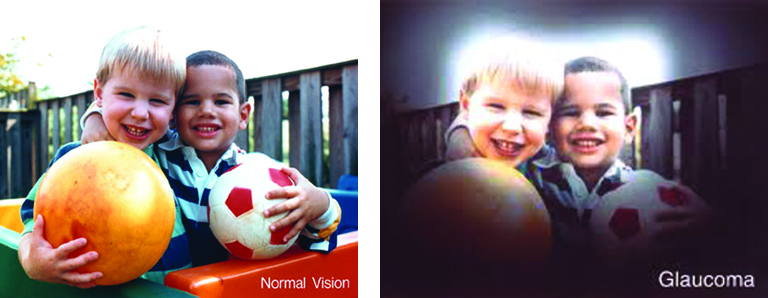
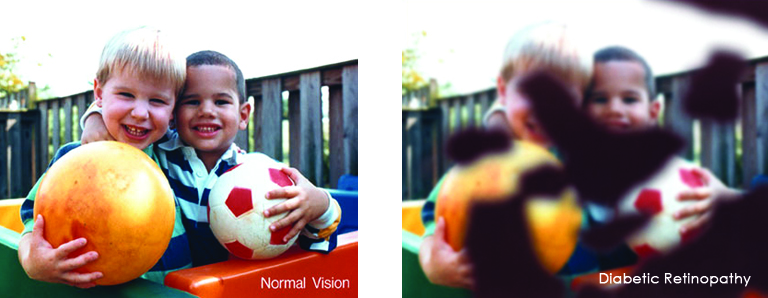
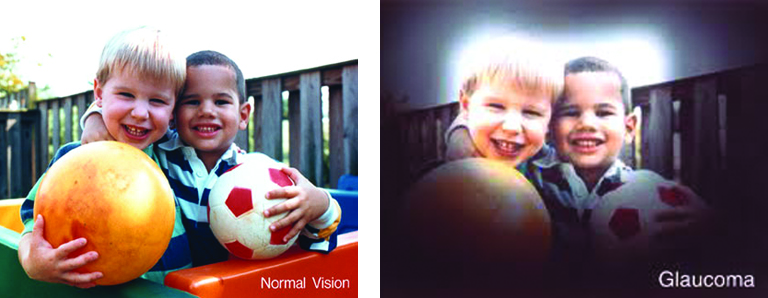
January is Glaucoma Awareness Month and the perfect time to raise awareness about this disease. Early on there are no symptoms. In fact, many people with glaucoma don’t even know they have it. Learn about glaucoma and the steps you can take to reduce your risk of vision loss.
There are several types of glaucoma, although the most common type of glaucoma is age-related Primary Open-Angle Glaucoma. It affects people starting at age 40, but can develop anytime later in life.
So, the way to detect if you have it is to get regular thorough eye examinations every two years after age 40.
ARE YOU AT RISK?
Anyone can get glaucoma, but certain groups are at higher risk. These groups include African Americans and Hispanics over the age of 40, and all people over the age of 60. Since it is hereditary, people with a family history of glaucoma and people who have diabetes are at higher risk.
4 GLAUCOMA GUIDELINES TO REMEMBER
1. Glaucoma can affect people of all ages – Although it is generally associated with seniors, glaucoma can strike anyone.
2. Demographics do play a role – Glaucoma is a leading cause of blindness among African Americans; it’s also highly prevalent in Hispanics over the age 65.
3. Is glaucoma hereditary? – The risk of developing primary open–angle glaucoma is up to nine times more likely if parents or siblings have the disease.
4. Hope for future glaucoma patients – Although there is no cure for glaucoma, early diagnosis and treatment can help control the disease and slow or stop the process of vision loss and blindness.
There are many steps you can take to help protect your eyes and lower your risk of vision loss from glaucoma.
- If you are in a high-risk group, get a comprehensive dilated eye exam every two years to catch glaucoma early and start treatment. Prescription eye drops, and if that fails, surgery can stop glaucoma from progressing.
- Even if you are not in a high-risk group, getting a comprehensive dilated eye exams can help catch glaucoma and other eye diseases early.
- Open-angle glaucoma does not have symptoms and is hereditary. So talk to your family members about their vision health to help protect your eyes and theirs.
- Maintaining a healthy weight, controlling your blood pressure, being physically active and avoiding smoking will help you avoid vision loss from glaucoma.
- Stay aware of the risks and symptoms and remember an annual comprehensive eye exam is key for early detection of glaucoma and other eye diseases.
Click here to get more information on Glaucoma.
Discovery Eye Foundation research is undertaking a new approach to save vision for glaucoma patients.
Read our 2022 Thanksgiving Newsletter to learn more.
CLICK LINK BELOW IF YOU WOULD LIKE TO HELP SUPPORT DEF’S GLAUCOMA RESEARCH


 Harsh weather conditions can reduce the natural moisture in your eyes and the irritation usually results in a burning or itching sensation that often leads to rubbing or scratching your eyes which can worsen the symptoms. Sometimes it feels like there is a foreign object in your eye and for some, dry eyes can even cause excessive tearing, as your eyes try to overcompensate for their lack of protective tears. Prolonged, untreated dry eyes can lead to blurred vision as well. Between the harsh winter winds outside and the dry heat radiating inside, our eyes are very quickly irritated and dried in the winter months. The result is itchy, dry eyes that may cause pain, blurred vision, a burning sensation, or even watery vision as our eyes try to compensate for the dryness.
Harsh weather conditions can reduce the natural moisture in your eyes and the irritation usually results in a burning or itching sensation that often leads to rubbing or scratching your eyes which can worsen the symptoms. Sometimes it feels like there is a foreign object in your eye and for some, dry eyes can even cause excessive tearing, as your eyes try to overcompensate for their lack of protective tears. Prolonged, untreated dry eyes can lead to blurred vision as well. Between the harsh winter winds outside and the dry heat radiating inside, our eyes are very quickly irritated and dried in the winter months. The result is itchy, dry eyes that may cause pain, blurred vision, a burning sensation, or even watery vision as our eyes try to compensate for the dryness.
 Make sure they take frequent screen breaks. Instead of focusing directly on the screen, encourage your child to look around the room every now and then, or take some time to stare out the window (at least 20 seconds is recommended by the American Optometric Association). You can even remind them to blink.
Make sure they take frequent screen breaks. Instead of focusing directly on the screen, encourage your child to look around the room every now and then, or take some time to stare out the window (at least 20 seconds is recommended by the American Optometric Association). You can even remind them to blink. It’s easy for us to forget about our eyes let alone our child’s, but it is very important to get your child’s eyes checked regularly.
It’s easy for us to forget about our eyes let alone our child’s, but it is very important to get your child’s eyes checked regularly. FIREWORK & EYE SAFETY:
FIREWORK & EYE SAFETY:  Even sparklers can be dangerous, as they burn at more than 2,000 degrees Fahrenheit. Sparklers were responsible for 900
Even sparklers can be dangerous, as they burn at more than 2,000 degrees Fahrenheit. Sparklers were responsible for 900  Most people have eye problems at one time or another. Some are minor and will go away on their own, or are easy to treat at home. Others need a specialist’s care. Some eye issues come with age while others may be a serious condition.
Most people have eye problems at one time or another. Some are minor and will go away on their own, or are easy to treat at home. Others need a specialist’s care. Some eye issues come with age while others may be a serious condition. Dry eye is a common condition that occurs when your tears aren’t able to provide adequate lubrication for your eyes. Tears can be inadequate for many reasons. For example, dry eyes may occur if you don’t produce enough tears or if you produce poor-quality tears. Dry eyes can also feel very uncomfortable.
Dry eye is a common condition that occurs when your tears aren’t able to provide adequate lubrication for your eyes. Tears can be inadequate for many reasons. For example, dry eyes may occur if you don’t produce enough tears or if you produce poor-quality tears. Dry eyes can also feel very uncomfortable.
 As winter shifts to spring, and flowers, grasses and trees begin to bloom, spring can take a toll on your eyes if you suffer from seasonal allergies. The spring season has a marked an increase in pollen and allergens in the air, that leave you with congestion, headaches, and itchy, swollen eyes, known as eye allergies.
As winter shifts to spring, and flowers, grasses and trees begin to bloom, spring can take a toll on your eyes if you suffer from seasonal allergies. The spring season has a marked an increase in pollen and allergens in the air, that leave you with congestion, headaches, and itchy, swollen eyes, known as eye allergies.