Similar Diseases, Different Locations, Possible Common Treatments
There are many similarities between two age-related diseases (Age-related Macular Degeneration and Alzheimer’s disease) that can affect thousands of people world-wide. In the United States there are 11 million people that have some form of AMD and it is estimated to grow to 22 million by the year 2050. Furthermore, in 2016 it was estimated that the cost to care for those with AMD was $512 billion. Worldwide it is estimated that by 2020, there will be 96 million people with AMD. National Eye Institute
The second aging disorder that causes high degree of damage is Alzheimer’s disease. Presently, in the United States there are 5.4 million people with Alzheimer’s disease and this will increase to approximately 13.8 million by 2050. In 2016, the cost for caring for these patients was $236 billion. Worldwide the numbers of Alzheimer’s patients are estimated to be 44 million and the global cost is $605 billion. Alzheimer’s Association
Similar Risk Factors
The risk factors for both AMD and Alzheimer’s disease are very similar to each other. These include aging, smoking, and high cholesterol. Both diseases are found more frequently in women than men and in approximately 5% to 15% the diseases are found in more than one family member. There is also a genetic risk factor of a lipid transport protein called Apolipoprotein E (ApoE) that provides elevated risk in AMD patients if they carry the allele 2 variant and higher risk in Alzheimer’s patients if they carry the allele 4 variant.
In AMD and Alzheimer’s disease there are 3 events that make the pathologies very similar except that they are found in different locations, either the retina or the brain.
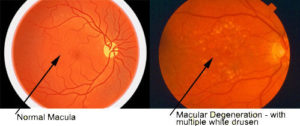
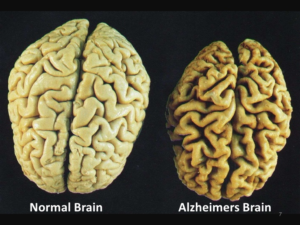
1. Amyloid beta is a protein that is not present in normal tissues but larger quantities accumulate in the brain for Alzheimer’s patients and are identified to be plaques by MRI scans. The presence of these plaques is defining (pathognomonic) for Alzheimer’s disease. In AMD patients amyloid-beta deposits are found to accumulate underneath the retina and form small clumps of protein-lipid materials called drusen. This is significant because the amyloid-beta is very toxic and harmful to the surrounding cells and when it is accumulating in tissues, it causes the cells to be damaged and loss their abilities to function.
2. A second feature of both AMD and Alzheimer’s disease is that there are high levels of tissue damage, loss of function and a lot of cell death in the retina and brain.
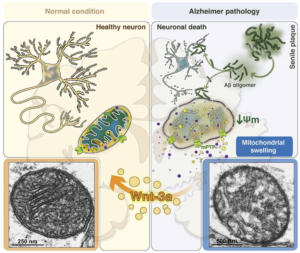
3. Finally, both diseases have damage to the mitochondria, which are small units within the cells that are critical to keeping the cells alive. The mitochondria are the “batteries” of the cell providing energy to keep the retina and brain cells functioning. Mitochondria are similar to the batteries in a flashlight. You can have a very expensive flashlight but if you do not have good batteries, the flashlight will not work. It is a similar situation to the cell. As long as the mitochondria are healthy and providing energy the cells can function. However, when the mitochondria start to die, then the cells will lose their functions and cell death will occur. This is true for all types of cells in the body, such as nerve cells, muscle cells, retinal cells, heart cells, etc. In other words, healthy mitochondria are critical to keep cell alive and functioning well.
Future Treatments
Using a novel in vitro model called cybrids (cytoplasmic hybrids), Dr. Cristina Kenney’s laboratory has shown that when mitochondria from patients with AMD are placed into specialized human retinal cells, the AMD mitochondria will cause the cells to die more rapidly than normal because they are so damaged. With this important discovery, the goal of the research group has been to identify drugs and proteins/peptides that can rescue the damage AMD mitochondria and protect the retinal cells. Their research is moving forward very quickly and testing drugs is the top priority for Dr. Kenney’s group. By rejuvenating the mitochondria from ‘old-damage’ to ‘new-healthy’ will prolong the health of the retinal cells and protect vision loss from AMD. What is learned in these studies will have long reaching applications to other aging-diseases such as Alzheimer’s and Parkinson’s diseases.